

The molecular mass is the relative mass of a molecule compared with the group of a carbon-12 atom taken as 12 units. We have studied that the mass of the atoms of an element is known as its atomic mass. Learn Average Molecular Mass What is Molecular Mass? The isotope of the carbon atom is chosen as a standard atom because it is solid and can be handled easily.Īvogadro, in 1811 suggested that the atomic and molecular masses can be expressed on an atomic mass scale based on the comparison of atomic/ molecular masses with the mass of a reference atom. To overcome this difficulty, the chemists determine the masses of these particles by comparing them with a mass of carbon atoms. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula.Molecular Mass: Atoms and molecules are so tiny that it is difficult to calculate their mass. When calculating molecular weight of a chemical compound, it tells us how many grams are in one mole of that substance. ELEMENT CHEMICAL ATOMIC MOLAR ELEMENT CHEMICAL ATOMIC MOLAR NAME SYMBOL NUMBER MASS NAME SYMBOL NUMBER MASS Actinium Ac 89 (227) Mendelevium Md 101 (258) Aluminum Al 13 26.982 Meitnerium Mt 109 (266) Americium Am 95 (243) Mercury Hg 80 200.59 Antimony Sb 51 121.75 Molybdenum Mo 42 95.94. The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom (or group of atoms) in the formula by the formula weight and multiplying by 100.įinding molar mass starts with units of grams per mole (g/mol). If the formula used in calculating molar mass is the molecular formula, the formula weight computed is the molecular weight.
F MOLAR MASS HOW TO
This site explains how to find molar mass. The reason is that the molar mass of the substance affects the conversion.
To complete this calculation, you have to know what substance you are trying to convert. See also our theoretical yield calculator for chemical reactions (probably your next stop to finish the problem set). These relative weights computed from the chemical equation are sometimes called equation weights.Ī common request on this site is to convert grams to moles. The molar mass of CF2Cl2 (Freon) is: 120.907 grams/mol. For bulk stoichiometric calculations, we are usually determining molar mass, which may also be called standard atomic weight or average atomic mass.įormula weights are especially useful in determining the relative weights of reagents and products in a chemical reaction. This is not the same as molecular mass, which is the mass of a single molecule of well-defined isotopes. It is found to contain 40.00 carbon, 6. For example, a molecule has a molecular weight of 180.18 g/mol. If you are given percent composition, you can directly convert the percentage of each element to grams. Determine the mass in grams of each element in the sample.
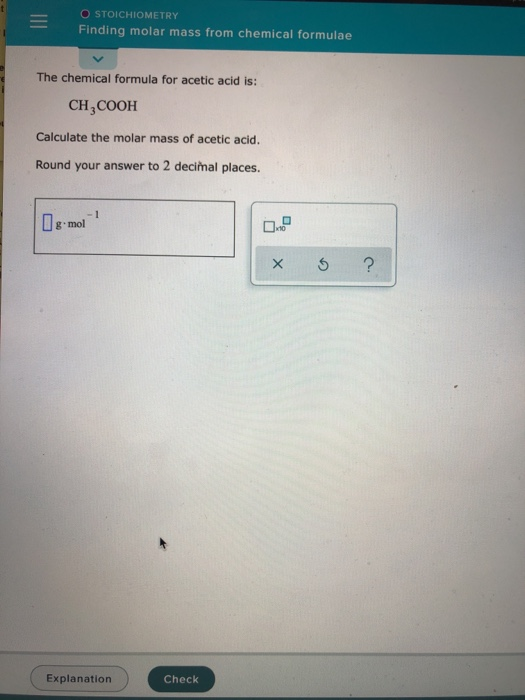
This is how to calculate molar mass (average molecular weight), which is based on isotropically weighted averages. You start by determining the empirical formula for the compound. The atomic weights used on this site come from NIST, the National Institute of Standards and Technology. Using the chemical formula of the compound and the periodic table of elements, we can add up the atomic weights and calculate molecular weight of the substance. In chemistry, the formula weight is a quantity computed by multiplying the atomic weight (in atomic mass units) of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together.


 0 kommentar(er)
0 kommentar(er)
